Interesting point Lebanon, and shows insight into Trogs thought process. I gather that he is a staunch union supporter, not that there is anything necessarily wrong with that, my Dad was in one all of his working life, but only because he had to be. An assumption to be sure, but I'll bet he faithfully pays his union dues hoping it will benefit him, while ignoring his SN dues, because he sees no personal benefit in doing so.No they have a keen understanding of politics and who to kiss up to..
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Covid Vaccine
- Thread starter jitter77
- Start date
- Joined
- Sep 12, 2014
- Messages
- 17,121
- Reaction score
- 6,753
- Points
- 113
That says the vaccine and “shot” are one in the same. People here are insisting that the vaccine is not a vaccine, rather its ONLY a “shot” and should be referred to ONLY as such. I’m asking WTF are they talking about?They don't, but it's only common sense.
The flu vaccine is more commonly referred as the flu shot.
The flu shot doesn't guarantee you don't get the flu either.
From the CDC site:
What is a flu vaccine?
Influenza (flu) vaccines (often called “flu shots”) are vaccines that protect against the four influenza viruses that research indicates most common during the upcoming season. Most flu vaccines are “flu shots” given with a needle, usually in the arm, but there also is also a nasal spray flu vaccine.
The composition of flu vaccines has been updated for the 2021-2022 flu season.
Is there more than one type of flu shot available?
Yes. There are different flu vaccine manufacturers and multiple influenza vaccine products licensed and recommended for use in the United States.
An annual seasonal flu vaccine is the best way to help protect against flu. Vaccination has been shown to have many benefits including reducing the risk of flu illnesses, hospitalizations and even the risk of flu-related death in children. While some people who get a flu vaccine may still get sick, flu vaccination has been shown in several studies to reduce severity of illness.
Sounds a lot like COVID.
- Joined
- Sep 12, 2014
- Messages
- 17,121
- Reaction score
- 6,753
- Points
- 113
No, Tim. I’m only inferring the existence of anti-mandate laws in 2021 (and beyond) will lead to increased deaths.LMAO.
You actually just said in 2020 there were no laws against vaccine mandates...when....there.....were....no...vaccines


Inferring that the lack of those laws against mandates against something that didn't exist had some material effect on the outcome LOL.
You actually also inferred that "outlawing" mandates also had some material effect in 2021. When Florida is above the national average in vaccinations AND he didn't ban mandates untillllllllll September LMAO


Has it been said before you really suck at this? Clearly not enough.
Many Floridians had come to Jesus moments in August and September and got vaccinated. Unfortunately, it looks like many others never will. Some will certainly regret it.

Florida COVID vaccinations plummet as delta variant toll wanes
Infections and deaths continue to decline, but first-dose vaccinations haven’t been this low since January.
Thats because according to the definition of a vaccine: "A vaccine is a biological preparation that provides active acquired immunity to a particular infectious disease", the COVID vaccine ain't cutting it.That says the vaccine and “shot” are one in the same. People here are insisting that the vaccine is not a vaccine, rather its ONLY a “shot” and should be referred to ONLY as such. I’m asking WTF are they talking about?
This vaccine doesn't provide immunity. I personally know 3 vaxed people who contracted it, but had little to no symptoms.
Real vaccines provide immunity to diseases such as chicken pox and polio. All the COVID vax seems to do is alleviate the symptoms.
The sticking point is what the heck do you call it?
A symptom reducer? An alleviator?
Last edited:
No, Tim. I’m only inferring the existence of anti-mandate laws in 2021 (and beyond) will lead to increased deaths.
Many Floridians had come to Jesus moments in August and September and got vaccinated. Unfortunately, it looks like many others never will. Some will certainly regret it.

Florida COVID vaccinations plummet as delta variant toll wanes
Infections and deaths continue to decline, but first-dose vaccinations haven’t been this low since January.www.tampabay.com
No dumbass, in your own attempts to play "gotya" you made some of your stupidest claims yet.
Floggy - 2020: "No laws against vaccines mandates. None. "
How relieved we all are that Desantis had no laws to protect us against evil mandates for vaccines that didn't exist in 2020.
Floggy - 2021: " Laws against vaccine mandates implemented."
LMAO, implemented in september, 15 days ago ROFL.....but it's caused death everywhere run!!!!!!!!
That says the vaccine and “shot” are one in the same. People here are insisting that the vaccine is not a vaccine, rather its ONLY a “shot” and should be referred to ONLY as such. I’m asking WTF are they talking about?
The fact that you don't understand the differences between a vaccine and a shot surprises literally no one.
Do you understand the concept of allergy shots? I'm curious...
Provide your own commentary. Ummm....
Imagine how safe they'd feel if they each lost about 60lbs.
The pandemic lifestyle....
- Joined
- Apr 9, 2014
- Messages
- 23,424
- Reaction score
- 28,064
- Points
- 113
No they have a keen understanding of politics and who to kiss up to..
Bingo!
60! Try over 100.Imagine how safe they'd feel if they each lost about 60lbs.
Great news
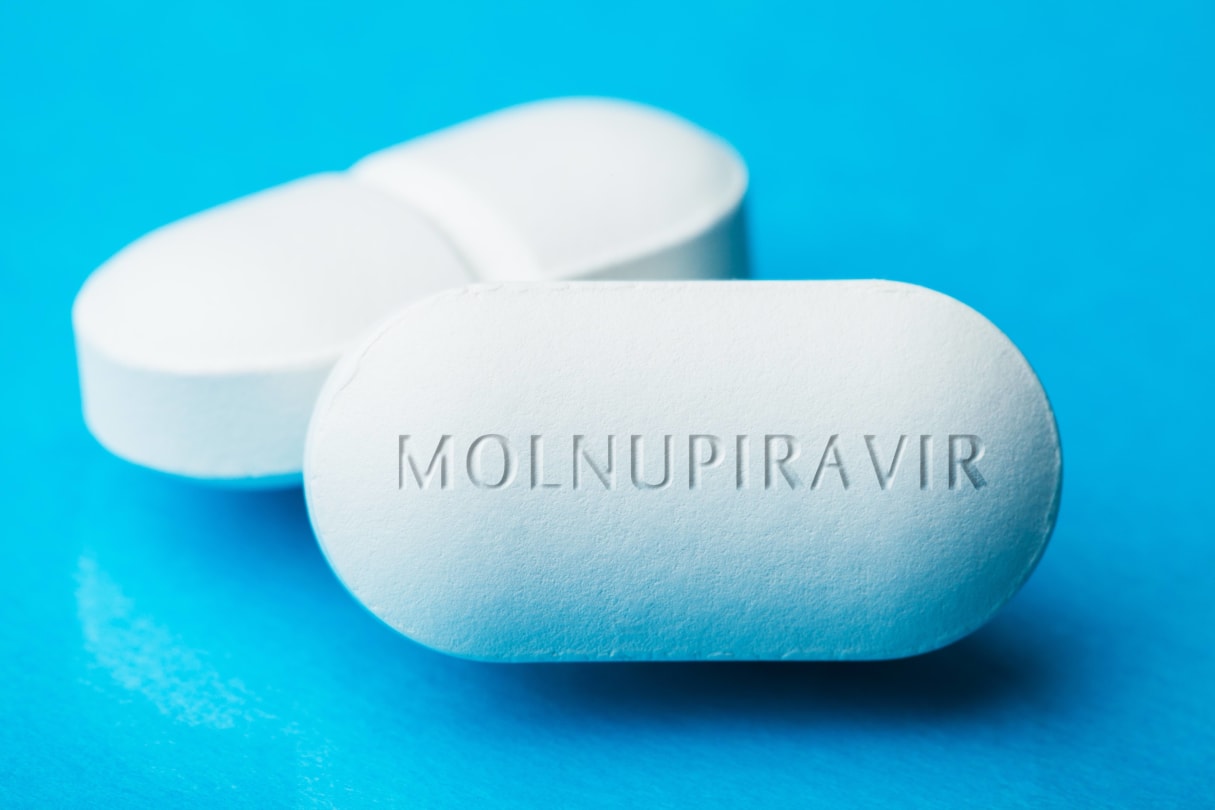
9 Things You Need To Know About the New COVID-19 Pill
A new pill from Merck called molnupiravir could be game-changer in the battle against COVID-19. The company reported that the drug cut the risk of hospitalization and death by half in patients who had mild-to-moderate COVID-19.
More good news, the J & J vaccine has been shown to be just a effective as one of those circular band aids.

Covid boosters: Who needs them and how do they help?
A panel of advisers has just voted to recommend a Moderna booster - the latest on who's eligible.
www.bbc.com
Pfizer
Numbers: To date, more than 103 million US residents have been fully vaccinated with two Pfizer doses, while approximately 7 million have received boosters.Efficacy: Data shows that a full dosage of the Pfizer vaccine is 88% effective in preventing hospital admission. CDC data released in mid-September shows that the vaccine's effectiveness falls to 77% after 120 days.
Company Claim About Booster: Pfizer has been supportive of the need for boosters, with CEO Albert Bourla telling reporters that studies have shown that the vaccine's effectiveness steadily declines to about 84% for vaccinated people four to six months after receiving their second dose.
FDA Ruling: Pfizer boosters have been approved for older adults and 50 to 64 year olds with medical conditions, as well as adults with underlying medical conditions or those who live and work in high-risk settings.
Moderna
Numbers: To date, more than 69 million people have been fully vaccinated with the Moderna vaccine, with about 1.5 million people having received Moderna booster jabs.Efficacy: New data shows that Moderna's vaccine was about 93% effective at reducing the risk of being admitted to hospital with Covid-19. It stays about 92% effective after 120 days.
Company Claim About Booster: Last month, Moderna said that a half-dose booster jab would boost antibodies to a higher point than the initial two shots and believes a booster will be necessary "prior to the winter season". Currently, Moderna boosters have only been approved for certain people with weakened immune systems, such as cancer patients or transplant recipients.
FDA Ruling: On Thursday, a panel of FDA advisers unanimously voted to recommend a single half-dose booster for the elderly, the immunocompromised or those in high-risk jobs or living situations.
The FDA must now make a final decision on whether to authorise Moderna boosters. If authorised by the agency, a panel of CDC advisers will meet to discuss who will receive them.
Johnson & Johnson
Numbers: Nearly 15 million US residents have received a Johnson & Johnson (J&J) vaccine, which is administered in one dose. CDC data shows that only about 9,800 people have so far received J&J boosters.Efficacy: Research shows that the J&J vaccine is 71% effective in preventing the need for hospital care. After just 28 days, the vaccine's effectiveness falls to 68%.
Company Claim About Booster: Like Moderna, J&J has submitted a request for emergency use authorisation for its booster jab. In late September, the company said that research shows that a booster provides a nine-fold increase in antibodies. Four weeks later, it had climbed to a 12-fold increase.
FDA Ruling: On Friday, a panel of FDA advisers unanimously voted to recommend boosters for the one-shot J&J vaccine. The panel recommended that boosters be given to anyone over 18, at least two months after the initial dose.
- Joined
- Apr 8, 2014
- Messages
- 11,900
- Reaction score
- 16,898
- Points
- 113
Covid is Superman and the flu us Clark KentThey don't, but it's only common sense.
The flu vaccine is more commonly referred as the flu shot.
The flu shot doesn't guarantee you don't get the flu either.
From the CDC site:
What is a flu vaccine?
Influenza (flu) vaccines (often called “flu shots”) are vaccines that protect against the four influenza viruses that research indicates most common during the upcoming season. Most flu vaccines are “flu shots” given with a needle, usually in the arm, but there also is also a nasal spray flu vaccine.
The composition of flu vaccines has been updated for the 2021-2022 flu season.
Is there more than one type of flu shot available?
Yes. There are different flu vaccine manufacturers and multiple influenza vaccine products licensed and recommended for use in the United States.
An annual seasonal flu vaccine is the best way to help protect against flu. Vaccination has been shown to have many benefits including reducing the risk of flu illnesses, hospitalizations and even the risk of flu-related death in children. While some people who get a flu vaccine may still get sick, flu vaccination has been shown in several studies to reduce severity of illness.
Sounds a lot like COVID.
The Evolution of Narrative as Vaccines Fail to Deliver on Promises
View attachment 6709
View attachment 6710
View attachment 6711
View attachment 6712
View attachment 6713
View attachment 6714
View attachment 6715
View attachment 6716
View attachment 6717
View attachment 6718
I need to see more proof.
- FloggedOverandOver
- Joined
- Sep 12, 2014
- Messages
- 17,121
- Reaction score
- 6,753
- Points
- 113
UK to Fauci — We have a big problem…
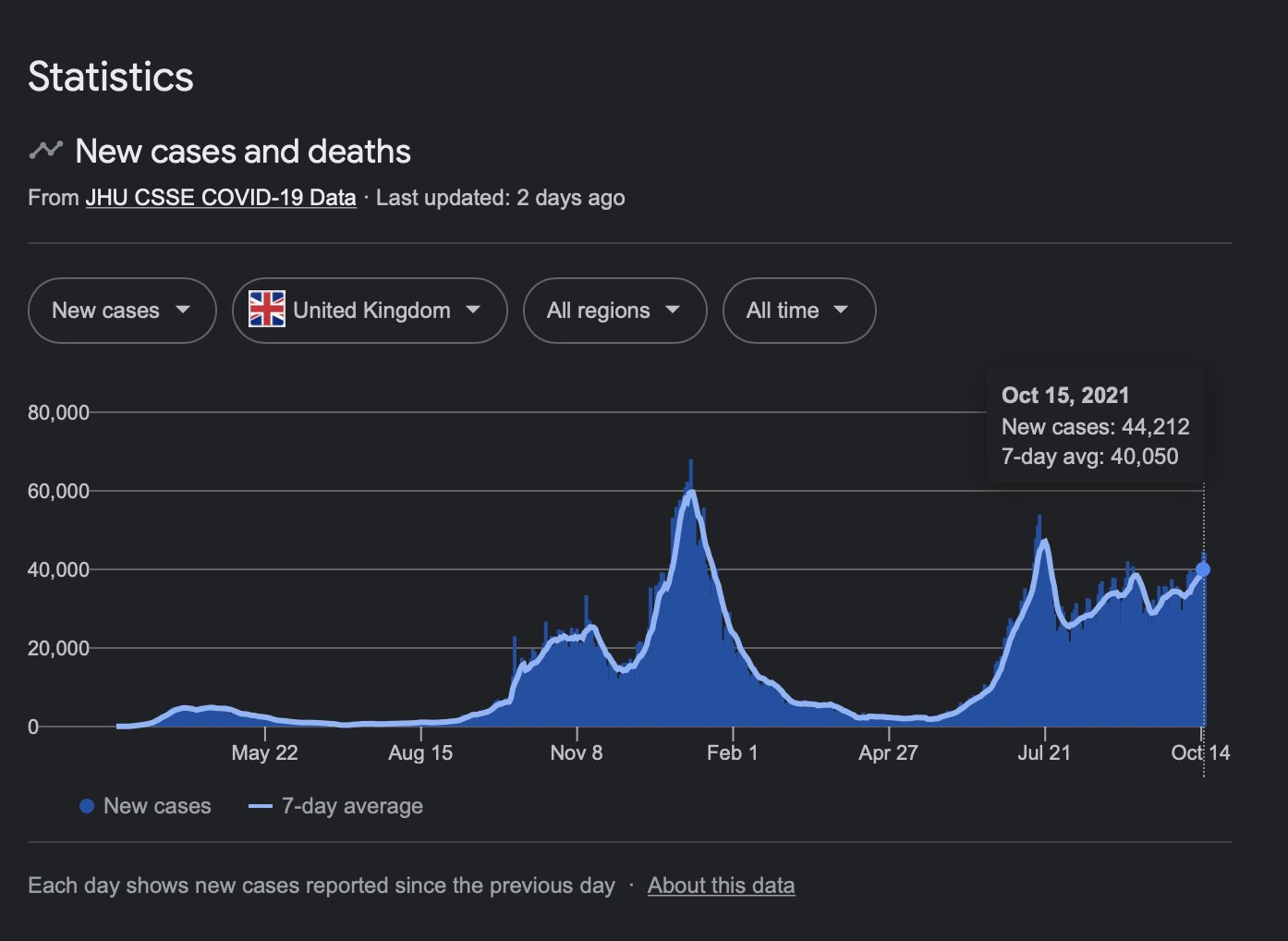
Cases are exploding despite the very high Vaccination rate
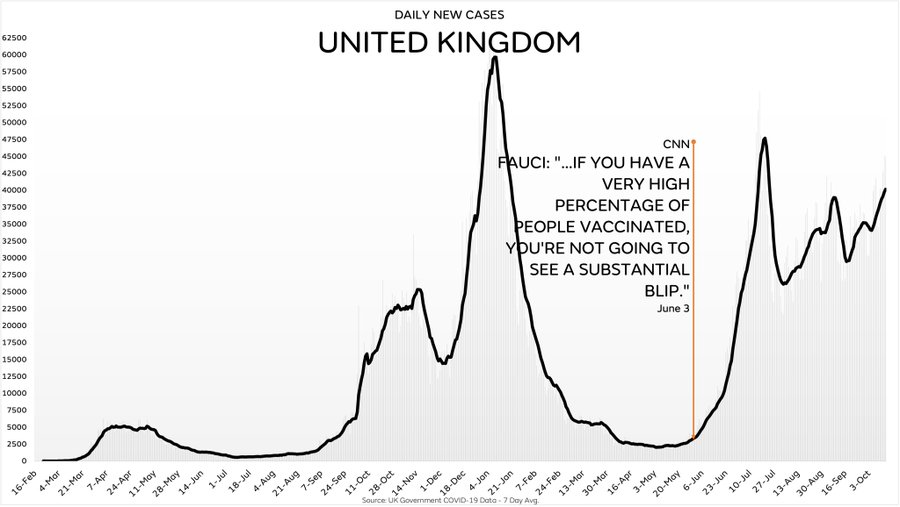
"The vaccines work, JFC!!!" /Floggy
UK is doing a helluva lot better than Florida, only 119 Covid deaths compared to Florida’s 170 at over 3X the population.
UK is doing a helluva lot better than Florida, only 119 Covid deaths compared to Florida’s 170 at over 3X the population.
Straw man. We are talking about the lack of effectiveness of the shots. England is evidence.
--------------------
Still see you are playing United "States" Roulette. Today it's Florida? Last week WV? Next week, ya moving back to South Dakota?
Currently, Florida is averaging 12 cases per 100K. #2 in the nation for lowest cases per 100K, behind only Hawaii.
Every, single Liberal, vaccinated state you lap the nuts of is doing worse than Florida.
NH: 40 per 100K
DE: 38 per 100K
VT: 38 per 100K
PA: 35 per 100K
ME: 30 per 100K
RI: 26 per 100K
NY: 23 per 100K
MA: 20 per 100K
And the UK??

It's not said often enough, but you suck at this.
16 year-old patient with no underlying conditions suffered after the mRNA Vaccine.
- Joined
- Sep 12, 2014
- Messages
- 17,121
- Reaction score
- 6,753
- Points
- 113
If people arent being hospitalized and dying, the vaccine is working. UK .18 deaths/100k, Florida .81 deaths/100k.Straw man. We are talking about the lack of effectiveness of the shots. England is evidence.
--------------------
Still see you are playing United "States" Roulette. Today it's Florida? Last week WV? Next week, ya moving back to South Dakota?
Currently, Florida is averaging 12 cases per 100K. #2 in the nation for lowest cases per 100K, behind only Hawaii.
Every, single Liberal, vaccinated state you lap the nuts of is doing worse than Florida.
NH: 40 per 100K
DE: 38 per 100K
VT: 38 per 100K
PA: 35 per 100K
ME: 30 per 100K
RI: 26 per 100K
NY: 23 per 100K
MA: 20 per 100K
And the UK??
View attachment 6722
It's not said often enough, but you suck at this.
If people arent being hospitalized and dying, the vaccine is working. UK .18 deaths/100k, Florida .81 deaths/100k.
Negative. Vaccines prevent cases. These
People are being hospitalized in the UK at an increasing rate since June 1:
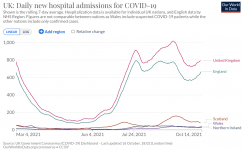
More people are dying since June 1:
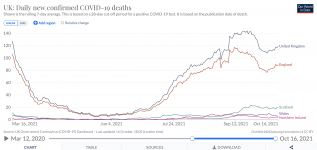
Yes, the UK is a poster-child case for the effectiveness of the vaccines.