and, what was your little graph informing us? how does the information in the graph support your stance? does the graph effectively dissuade anyone from believing differently about whatevergoddamneddata you're unsuccessfully attempting to argue?nytimes.com = New York Times. It’s a widely read newspaper for grownups.
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
The Coronavirus thread
- Thread starter hamster
- Start date
- Joined
- Apr 9, 2014
- Messages
- 19,962
- Reaction score
- 33,688
- Points
- 113
- Location
- $62.75 would help feed Paxton
You only understand pictures, huh?
Below the graph: 14 Day Change: +14%
Huh...When I go to the CDC it says the number are....down. It's almost like someone cherry picked a graph from a ****** lying news paper.
https://covid.cdc.gov/covid-data-tracker/#trends_dailytrendscases

Here's a little tidbit that I found interesting....
What is the average life expectancy in the U.S. of A?
Now, what is the median age of deaths in the country from the Chinese flu?
Hint, question #2 is higher.
What is the average life expectancy in the U.S. of A?
Now, what is the median age of deaths in the country from the Chinese flu?
Hint, question #2 is higher.
- Joined
- Apr 8, 2014
- Messages
- 13,807
- Reaction score
- 6,387
- Points
- 113
I think you forget which forum you’re posting in. There are no grownups here. If anything, that should be abundantly clear.nytimes.com = New York Times. It’s a widely read newspaper for grownups.
you sniveling little weasel.I think you forget which forum you’re posting in. There are no grownups here. If anything, that should be abundantly clear.
even YOU should acknowledge that graph meant jackshit
What is the average life expectancy in the U.S. of A?
Now, what is the median age of deaths in the country from the Chinese flu?
Hint, question #2 is higher.
LOL! Are YOU some sort of medical professional?!
- FloggedbyFauci™
There are no grownups here. If anything, that should be abundantly clear.
If that's why how you feel, you know there's a simple solution to that, correct? I mean we all understand you're an unhinged lunatic, but at least this much is clear to you, right?
- Joined
- Sep 12, 2014
- Messages
- 17,041
- Reaction score
- 6,722
- Points
- 113
From the link YOU provided…Huh...When I go to the CDC it says the number are....down. It's almost like someone cherry picked a graph from a ****** lying news paper.
https://covid.cdc.gov/covid-data-tracker/#trends_dailytrendscases
Daily Case Trends 7 Day Average
6/20/21: 11,378
7/6/21: 13,859
case dismissed.


Daily Case Trends 7 Day Average
6/20/21: 11,378
7/6/21: 13,859
Good god, you really are a feckless little *****. How about we look at the entire graph?
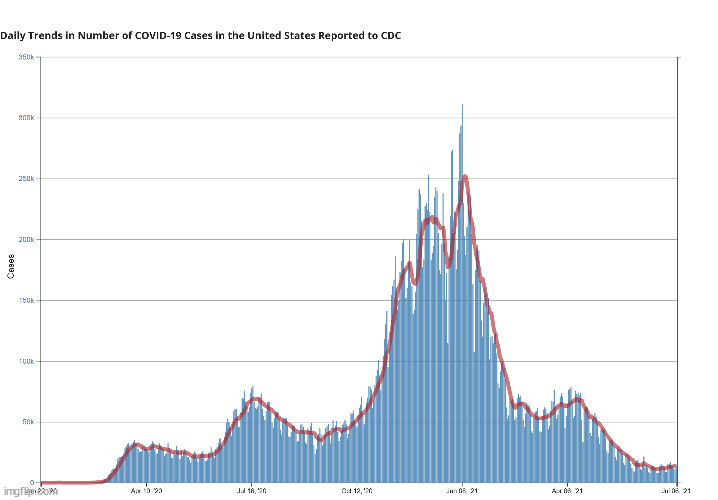
Gee, that looks a bit like a seasonal flu graph. And which way has it been trending for months and months?

Searching for FloggedbyFauci's™ nutsack.

my point exactly!!!
- Joined
- Sep 12, 2014
- Messages
- 17,041
- Reaction score
- 6,722
- Points
- 113
Cases are rising. Obviously, Covid isn’t over and we haven’t reached 96% herd immunity like Tim and you idiotically insist.and, what was your little graph informing us? how does the information in the graph support your stance? does the graph effectively dissuade anyone from believing differently about whatevergoddamneddata you're unsuccessfully attempting to argue?
LOL! 319 Million. Check that, 284 Million. Check that 196 Million. How many revisions until you realize that you have no ******* business making assumptions followed by math? Put your visceral conviction aside, think things through, then post.
And your 161 Million is yet another extrapolation. How about you back up your “1,100 kids have died from the vaccine” assertion first before you start with any more extrapolations?
Floggy, you don't even realize I'm playing your stupid game. Each time I posit numbers that are supported, you say "what about." So I play "what about."
I've taken every curve ball you've attempted to throw my way. Even if I let you strip out every single little thing you can come up with, we undeniably have over 161Million Americans with antibodies to the virus. At a minimum over half of the USA is protected against COVID.
Now, as I said, add in those vaccinated that don't fit into that population. Add half of those vaccinated. I don't care. That still takes you to 240Million Americans protected against the virus.
This is why COVID is no longer an issue, cases are down, deaths are basically a statistical anomaly.
Your data is a bunch of nonsensical extrapolations and assumptions. The proof that they’re wrong is the FACT that new daily cases are again on the rise and no qualified health official is claiming anything close to what you are claiming. It’s nonsense, Tim.
LOL, now we have HypoFlog on record denying supportable, government-supplied facts.
The CDC did publish that for every COVID case diagnosed, there are 4.6 more out there that went undiagnosed.
The government has tracked and shown that 35 Million Americans were infected with COVID.
The government has tracked and shown that now 158Million Americans are vaccinated.
But HypoFlog do be say these are downright lies because....ideology.
**** you simply can't make up

Meanwhile, HypoFlog and Tibs be saying we need to vaccinate all children.
Good to be right. Again.

 www.medpagetoday.com
www.medpagetoday.com

Last week, the CDC's Advisory Committee on Immunization Practices (ACIP) met to discuss the safety signal of myocarditis among young people who receive mRNA vaccination against COVID-19. This dialogue has been months in the making. Ultimately, the panel continued to endorse a two-dose mRNA strategy for all ages. We are concerned with this recommendation and offer five alternative considerations. But first, let's review how we got to this moment in order to make sense of vaccine-induced myocarditis.
A Recent History of Vaccine-Induced Myocarditis
The potential risk for vaccine-induced myocarditis was first raised on February 1 in the Jerusalem Post, which reported the hospitalization and intensive-care admission of a healthy 19-year old male 5 days after receiving his second dose of the Pfizer vaccine. This was followed by a nationwide report in the Times of Israel on April 23, later picked up by Reuters on April 25. These news reports suggested that Israel had seen elevated rates of this event after young men were vaccinated with the Pfizer vaccine, almost always after the second dose (56 out of 62 cases or 90%).
The European Medicines Agency announced an investigation on May 7, which was the same day several of us cautioned against FDA's use of emergency use authorization (EUA) to expedite the availability of COVID-19 vaccines to U.S. kids ages 12 to 15.
Although they were aware of this safety signal, the FDA issued the EUA on May 10 for Pfizer's mRNA vaccine in kids ages 12 to 15. Despite the fact that the vaccine was already widely in use in people ages 16 and above under the existing EUA, specific rates of myocarditis in the U.S. for 'near age' vaccine recipients (kids ages 16 to 18) were not made publicly available. In other words, no data on myocarditis events in kids close in age to the group receiving the new EUA (those ages 12 to 15) were leveraged in the process for granting this EUA. This is unfortunate, as these data would have had the greatest relevance and implications for the adjacent-age group.
Over the last 2 months, several news reports on clusters of cases of myocarditis after mRNA vaccination -- particularly in young men -- have been reported in the U.S. Revised estimates from Israel found the rate of myocarditis to be to one in 3,000 to one in 6,000 among males ages 16 to 24. On May 26, the Times of Israel reported that Israel's health ministry would consider just one dose in teens to balance getting most of the benefit of viral protection against mitigating much of the risk of myocarditis.
Israel now recommends vaccinating kids 12 to 15, but other nations have been more cautious. The U.K. advisers have decided not to support vaccination for kids under 18. The Germany standing vaccination commission advised that only children with pre-existing conditions receive the vaccine. The Netherlands (Dutch) health counsel advised only kids with pre-existing conditions or those living in a household with a family member who cannot be vaccinated receive the vaccine.
On May 22, the CDC announced they had received reports of myocarditis, and asked healthcare providers to file additional and missing reports into the Vaccine Adverse Event Reporting System (VAERS).
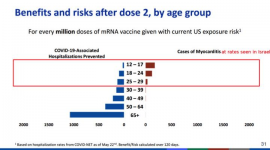
Last week, on June 23, ACIP met to discuss the findings. To date, CDC has documented myocarditis in at least 323 cases age 29 or under (of whom 96% were hospitalized), while 148 remain under review. The CDC acknowledged more cases in young people than older people, more cases in young males than young females, and higher incidence after dose two than dose one. The absolute risk of myocarditis after the second dose based on the number of CDC confirmed cases would be one in 15,000 to 20,000 for boys ages 12 to 24. There is a smaller but still excess risk in women age 24 and younger.
At its meeting, ACIP considered figures and data, which claimed to weigh the benefits versus harms of "dose two" of mRNA vaccines in this age group. However, in reality, the scenarios presented by the CDC compared the risks versus benefits to young people of no vaccination at all versus a scenario in which they received both shots.
The CDC did not consider the harms versus benefits of one versus two doses, but only the harms versus benefits of vaccination itself. But the CDC went beyond this. They also used base rates of infection from the past, rather than current rates of SARS-CoV-2 spread, which are substantially lower. They did not differentiate between healthy kids -- who are at risk of idiosyncratic events, such as myocarditis -- and kids with pre-existing medical conditions that place them at high risk of severe outcomes from COVID-19, including hospitalization.
This insistence on an all-or-nothing, one-size-fits-all binary approach -- treating healthy kids who have recovered from confirmed prior infection as equivalent to infection-naive kids with comorbidities -- is at the heart of the fallacy underpinning ACIP's decision.
While we acknowledge the CDC and ACIP had to act based on short-term studies and limited and variable data, vaccines must be used in a way that maximizes benefit and minimizes risk.
Ultimately, the CDC's recommendations came out so unequivocally in favor of vaccination that the following is true: If a 15-year-old recovers from COVID-19 and has high antibody levels, and this 15-year-old then receives one dose of mRNA vaccine causing hospitalization from myocarditis, the CDC would still contemplate proceeding with dose two once the "heart has recovered."
These events raise several points of concern:
Good to be right. Again.

CDC's All-or-Nothing Approach to Teen COVID Vaccination Is All Wrong
The agency should revisit its latest guidance to maximize benefits and minimize risks

Last week, the CDC's Advisory Committee on Immunization Practices (ACIP) met to discuss the safety signal of myocarditis among young people who receive mRNA vaccination against COVID-19. This dialogue has been months in the making. Ultimately, the panel continued to endorse a two-dose mRNA strategy for all ages. We are concerned with this recommendation and offer five alternative considerations. But first, let's review how we got to this moment in order to make sense of vaccine-induced myocarditis.
A Recent History of Vaccine-Induced Myocarditis
The potential risk for vaccine-induced myocarditis was first raised on February 1 in the Jerusalem Post, which reported the hospitalization and intensive-care admission of a healthy 19-year old male 5 days after receiving his second dose of the Pfizer vaccine. This was followed by a nationwide report in the Times of Israel on April 23, later picked up by Reuters on April 25. These news reports suggested that Israel had seen elevated rates of this event after young men were vaccinated with the Pfizer vaccine, almost always after the second dose (56 out of 62 cases or 90%).
The European Medicines Agency announced an investigation on May 7, which was the same day several of us cautioned against FDA's use of emergency use authorization (EUA) to expedite the availability of COVID-19 vaccines to U.S. kids ages 12 to 15.
Although they were aware of this safety signal, the FDA issued the EUA on May 10 for Pfizer's mRNA vaccine in kids ages 12 to 15. Despite the fact that the vaccine was already widely in use in people ages 16 and above under the existing EUA, specific rates of myocarditis in the U.S. for 'near age' vaccine recipients (kids ages 16 to 18) were not made publicly available. In other words, no data on myocarditis events in kids close in age to the group receiving the new EUA (those ages 12 to 15) were leveraged in the process for granting this EUA. This is unfortunate, as these data would have had the greatest relevance and implications for the adjacent-age group.
Over the last 2 months, several news reports on clusters of cases of myocarditis after mRNA vaccination -- particularly in young men -- have been reported in the U.S. Revised estimates from Israel found the rate of myocarditis to be to one in 3,000 to one in 6,000 among males ages 16 to 24. On May 26, the Times of Israel reported that Israel's health ministry would consider just one dose in teens to balance getting most of the benefit of viral protection against mitigating much of the risk of myocarditis.
Israel now recommends vaccinating kids 12 to 15, but other nations have been more cautious. The U.K. advisers have decided not to support vaccination for kids under 18. The Germany standing vaccination commission advised that only children with pre-existing conditions receive the vaccine. The Netherlands (Dutch) health counsel advised only kids with pre-existing conditions or those living in a household with a family member who cannot be vaccinated receive the vaccine.
On May 22, the CDC announced they had received reports of myocarditis, and asked healthcare providers to file additional and missing reports into the Vaccine Adverse Event Reporting System (VAERS).
Last week, on June 23, ACIP met to discuss the findings. To date, CDC has documented myocarditis in at least 323 cases age 29 or under (of whom 96% were hospitalized), while 148 remain under review. The CDC acknowledged more cases in young people than older people, more cases in young males than young females, and higher incidence after dose two than dose one. The absolute risk of myocarditis after the second dose based on the number of CDC confirmed cases would be one in 15,000 to 20,000 for boys ages 12 to 24. There is a smaller but still excess risk in women age 24 and younger.
At its meeting, ACIP considered figures and data, which claimed to weigh the benefits versus harms of "dose two" of mRNA vaccines in this age group. However, in reality, the scenarios presented by the CDC compared the risks versus benefits to young people of no vaccination at all versus a scenario in which they received both shots.
The CDC did not consider the harms versus benefits of one versus two doses, but only the harms versus benefits of vaccination itself. But the CDC went beyond this. They also used base rates of infection from the past, rather than current rates of SARS-CoV-2 spread, which are substantially lower. They did not differentiate between healthy kids -- who are at risk of idiosyncratic events, such as myocarditis -- and kids with pre-existing medical conditions that place them at high risk of severe outcomes from COVID-19, including hospitalization.
This insistence on an all-or-nothing, one-size-fits-all binary approach -- treating healthy kids who have recovered from confirmed prior infection as equivalent to infection-naive kids with comorbidities -- is at the heart of the fallacy underpinning ACIP's decision.
While we acknowledge the CDC and ACIP had to act based on short-term studies and limited and variable data, vaccines must be used in a way that maximizes benefit and minimizes risk.
Ultimately, the CDC's recommendations came out so unequivocally in favor of vaccination that the following is true: If a 15-year-old recovers from COVID-19 and has high antibody levels, and this 15-year-old then receives one dose of mRNA vaccine causing hospitalization from myocarditis, the CDC would still contemplate proceeding with dose two once the "heart has recovered."
These events raise several points of concern:
- VAERS is a suboptimal system. While the VAERS system was well-positioned to detect a rare and entirely unprecedented safety event (e.g., vaccine induced thrombocytopenia and thrombosis in the cerebral vessels), the system is suboptimal for elevations in naturally occurring health outcomes. Voluntary reporting requires a provider to make a mental link between vaccination and the outcome, and the mere fact that the CDC received more cases after coverage in the New York Times is evidence that VAERS failed to capture these events without prompting. This indicates cases may still be underreported: U.S. rates are likely a floor and not a ceiling. The meticulous tracking in Israel is likely closer to the real figure. Facing a factor-of-5 discrepancy between rates reported by Israel and the U.S., it is not prudent to simply assume that Israel is overcounting myocarditis, rather than the other way around.
- If you change even one assumption, the CDC's model tips. Using the CDC's own framework of risk and benefit, key differences tip the calculus. First, the comparison doesn't have to be either two doses or no doses. We can also consider just a single dose. Dose two is associated with greater rates of myocarditis, and one dose of an mRNA vaccine has strong protection (over 90% for severe outcomes) -- even against novel variants such as Delta. If you do this, the calculus tips. Second, building on this model, if one assumes rates of myocarditis documented in Israel, accepting the hypothesis that VAERS underestimates risk, it gets even worse. One of us (Wes Pegden, PhD) re-ran the CDC's analysis accounting for this, which shows that second dose vaccination is unfavorable at young ages. Finally, the CDC's analysis uses SARS-CoV-2 rates from the past -- when fewer adults were vaccinated. Rates might rise in the fall, but that's unclear.

Last edited:
Prior post Continued...
3. The CDC did not consider alternative strategies. The decision facing the CDC is not whether or not COVID-19 vaccination in children is generally a good idea. Most immediately, it is whether kids ages 12 to 15 should continue to receive second doses. A range of vaccination strategies are possible in children. Believing that COVID-19 vaccines can be valuable even for healthy children is different from thinking we cannot afford to proceed cautiously. Above all, it does not mean CDC should feel a need to stay the course with second doses whose marginal risks in teens appear likely to exceed their marginal benefits. Manufacturers could also reconsider the dose given to young people under 25 years old. Children's vaccination trials currently underway use lower doses than the adult studies; perhaps a lower or intermediate dose of vaccine could preserve most of the anti-COVID-19 benefit while avoiding the myocarditis risk. The CDC did not explore this option. And notably, dose optimization is an area of drug development for which there is a lot of room for improvement.
4. The CDC is not accounting for COVID-19 risk factors. Vaccination strategies for young people should be responsive to risk factors that place children at elevated risk of severe COVID-19 disease. While it is true that some cases of multisystem inflammatory syndrome in children (MIS-C) are idiosyncratic -- occur even in healthy kids -- the bulk of adolescent hospitalizations are among individuals with pre-existing risk factors. In contrast, the risk of myocarditis is entirely idiosyncratic and can strike anyone, including healthy adolescents at very low risk of severe disease. Vaccinating those at high COVID-19 risk, but not all young people, is a strategy that must be considered when balancing tradeoffs, as the harms versus benefits to healthy children are different than for kids with risk factors.
5. The CDC is not factoring in natural immunity. It is hard to believe that the risk benefit balance favors a 15-year-old young man who has recovered from COVID-19, and who has detectable antibodies, getting two doses of an mRNA vaccine. Such an individual is accepting a non-negligible risk of myocarditis, with limited upside in terms of decreased risk of severe infection. If the CDC recommends vaccination for these children, it is imperative they weigh benefits versus harms in precisely this population, which, to date, they have not presented.
Immediately after the ACIP meeting, various agencies and professional societies released a joint statement arguing that the benefit of vaccination far outweighs the risk in all age groups and demographics. Yet, our analysis suggests this is a premature conclusion. It relies on models that use outdated COVID-19 risk rates -- the on-the-ground rates in the moment are far lower, shifting the harm/benefit calculus to harm. It assumes two doses or none at all are the only options. It does not tailor recommendations by sex, natural immunity, or even comorbidities. We acknowledge there are individual and community level benefits to vaccination that extend beyond preventing hospitalizations and are an important part of the discussion. But these omissions from ACIP/CDC are problematic.
The stakes of this decision are no small thing. Even ACIP acknowledged there is a lot we still do not know about myocarditis after vaccination. There are additional cases being adjudicated, including serious ones, and there are no long-term follow-up studies yet to determine, for example, whether documented evidence of myocardial scar may portend an increased risk of arrhythmias. The ACIP and CDC discussion around vaccinating young teenagers, specifically boys, left out reasonably middle ground positions.
True vaccine proponents -- as all of us are -- understand the best thing we can do for vaccines is deploy them in a way that maximizes benefit and minimizes risk. This is crucial to protect health and also to ensure public confidence in the safety of vaccination. The current CDC guidance is not that. It needs to be revisited.
----------------
Try reading those articles HyopFlog. I know it's a lot of big words, but these medical doctors state what I have been saying...as have other countries who are NOT vaccinating the young....that vaccinating teens and lower is unnecessary and comes with unnecessary risk. Leave them alone.
Will you trust doctors? 3 or 4 other nations that side with me?
Doubt it.
3. The CDC did not consider alternative strategies. The decision facing the CDC is not whether or not COVID-19 vaccination in children is generally a good idea. Most immediately, it is whether kids ages 12 to 15 should continue to receive second doses. A range of vaccination strategies are possible in children. Believing that COVID-19 vaccines can be valuable even for healthy children is different from thinking we cannot afford to proceed cautiously. Above all, it does not mean CDC should feel a need to stay the course with second doses whose marginal risks in teens appear likely to exceed their marginal benefits. Manufacturers could also reconsider the dose given to young people under 25 years old. Children's vaccination trials currently underway use lower doses than the adult studies; perhaps a lower or intermediate dose of vaccine could preserve most of the anti-COVID-19 benefit while avoiding the myocarditis risk. The CDC did not explore this option. And notably, dose optimization is an area of drug development for which there is a lot of room for improvement.
4. The CDC is not accounting for COVID-19 risk factors. Vaccination strategies for young people should be responsive to risk factors that place children at elevated risk of severe COVID-19 disease. While it is true that some cases of multisystem inflammatory syndrome in children (MIS-C) are idiosyncratic -- occur even in healthy kids -- the bulk of adolescent hospitalizations are among individuals with pre-existing risk factors. In contrast, the risk of myocarditis is entirely idiosyncratic and can strike anyone, including healthy adolescents at very low risk of severe disease. Vaccinating those at high COVID-19 risk, but not all young people, is a strategy that must be considered when balancing tradeoffs, as the harms versus benefits to healthy children are different than for kids with risk factors.
5. The CDC is not factoring in natural immunity. It is hard to believe that the risk benefit balance favors a 15-year-old young man who has recovered from COVID-19, and who has detectable antibodies, getting two doses of an mRNA vaccine. Such an individual is accepting a non-negligible risk of myocarditis, with limited upside in terms of decreased risk of severe infection. If the CDC recommends vaccination for these children, it is imperative they weigh benefits versus harms in precisely this population, which, to date, they have not presented.
Immediately after the ACIP meeting, various agencies and professional societies released a joint statement arguing that the benefit of vaccination far outweighs the risk in all age groups and demographics. Yet, our analysis suggests this is a premature conclusion. It relies on models that use outdated COVID-19 risk rates -- the on-the-ground rates in the moment are far lower, shifting the harm/benefit calculus to harm. It assumes two doses or none at all are the only options. It does not tailor recommendations by sex, natural immunity, or even comorbidities. We acknowledge there are individual and community level benefits to vaccination that extend beyond preventing hospitalizations and are an important part of the discussion. But these omissions from ACIP/CDC are problematic.
The stakes of this decision are no small thing. Even ACIP acknowledged there is a lot we still do not know about myocarditis after vaccination. There are additional cases being adjudicated, including serious ones, and there are no long-term follow-up studies yet to determine, for example, whether documented evidence of myocardial scar may portend an increased risk of arrhythmias. The ACIP and CDC discussion around vaccinating young teenagers, specifically boys, left out reasonably middle ground positions.
True vaccine proponents -- as all of us are -- understand the best thing we can do for vaccines is deploy them in a way that maximizes benefit and minimizes risk. This is crucial to protect health and also to ensure public confidence in the safety of vaccination. The current CDC guidance is not that. It needs to be revisited.
----------------
Try reading those articles HyopFlog. I know it's a lot of big words, but these medical doctors state what I have been saying...as have other countries who are NOT vaccinating the young....that vaccinating teens and lower is unnecessary and comes with unnecessary risk. Leave them alone.
Will you trust doctors? 3 or 4 other nations that side with me?
Doubt it.
Last edited:
- Joined
- Sep 12, 2014
- Messages
- 17,041
- Reaction score
- 6,722
- Points
- 113
What curve balls? I’ve refuted your nonsense by pointing out its flawed assumptions over and over and over.Floggy, you don't even realize I'm playing your stupid game. Each time I posit numbers that are supported, you say "what about." So I play "what about."
I've taken every curve ball you've attempted to throw my way. Even if I let you strip out every single little thing you can come up with, we undeniably have over 161Million Americans with antibodies to the virus. At a minimum over half of the USA is protected against COVID.
Now, as I said, add in those vaccinated that don't fit into that population. Add half of those vaccinated. I don't care. That still takes you to 240Million Americans protected against the virus.
This is why COVID is no longer an issue, cases are down, deaths are basically a statistical anomaly.
I’ve refuted your nonsense by pointing out its flawed assumptions over and over and over.

- Joined
- Apr 9, 2014
- Messages
- 19,962
- Reaction score
- 33,688
- Points
- 113
- Location
- $62.75 would help feed Paxton
Floggy: "The country had 17 more people get the Delta India strain of the not-Chinese flu so we need to lock down and vaccinate, vaccinate and mask, mask and lock down until nobody - NOBODY - in the nation gets the not-Chinese flu!!"
Translation: Floggy ran into traffic for the first time in 16 months.
Translation: Floggy ran into traffic for the first time in 16 months.
- Joined
- Sep 12, 2014
- Messages
- 17,041
- Reaction score
- 6,722
- Points
- 113
Simply shocking.
But Tim says that not only have we reached herd immunity, but that we reached it back it March!
Provide proof that Tim said specifically that.But Tim says that not only have we reached herd immunity, but that we reached it back it March!
But Tim says that not only have we reached herd immunity, but that we reached it back it March!
Yeah, let's put stock in a graph from XiNN posted by this retard:

Who actually says this in his profile: "love human & animal the same way."
Dollars to donuts he's really just another Hungtard.


